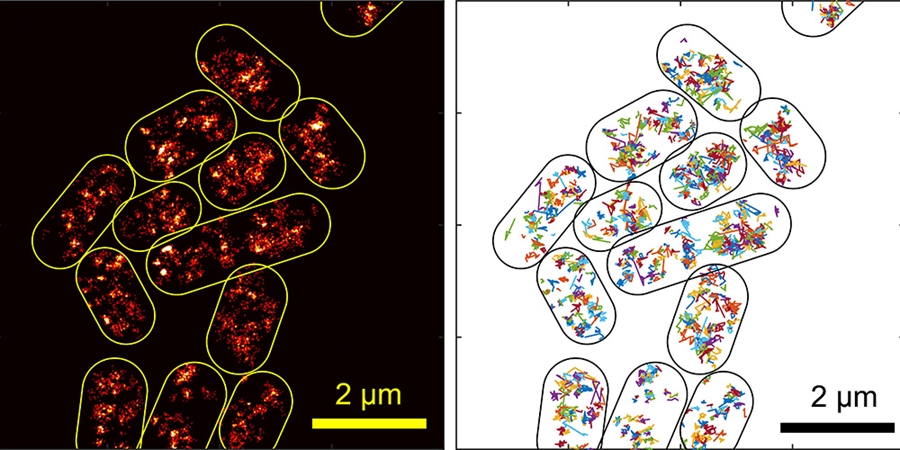
Yong Wang, assistant professor of physics, and graduate student Asmaa Sadoon have been studying how molecules travel through bacterial cytoplasm in order to understand more about how these tiny organisms function. Using new high-tech tools, they have been able to observe certain processes inside live bacteria for the first time. They published their results in the journal Physical Review E.
The researchers used a combination of super-resolution fluorescence microscopy and a technique called single-particle tracking to study how a type of protein called H-NS moves through the cytoplasm of E. coli cells. The researchers chose this protein because it interacts with both proteins and DNA, and it helps regulate gene expression in the bacteria. Understanding bacterial gene expression could lead to new techniques to mitigate bacterial resistance to antibiotics.
In this study, the researchers learned new information about this protein, and about the properties of bacterial cytoplasm. Wang describes cytoplasm as "a thick soup of proteins, DNA, and various other molecules." Because bacteria don't have transport systems, such as digestive or circulatory systems, they depend on the diffusion of molecules through this soup for the processes that keep them alive.
By tracking the movement of H-NS through the cytoplasm of the E. coli, the researchers were able to calculate the viscoelasticity of the cytoplasm. They found that the bacterial "soup" doesn't behave the same way a homogenous protein solution does.
Previous research, which used homogenous solutions studied in vitro, observed that in these solutions, both elasticity and viscosity decreased over time. In other words, the solutions became both thinner and softer. In actual bacteria, however, Wang and Sadoon observed that, after a certain time-scale, the viscosity, or thickness, of the cytoplasm flattens out, so the bacterial cytoplasm gets softer without getting thinner.
"Our findings are expected to fundamentally change the way bacterial cytoplasm is viewed," the researchers explained in the paper. "Unlike a simple viscous or viscoelastic fluid that current models of bacterial processes typically consider, the bacterial cytoplasm behaves differently at different time scales in terms of mechanical properties, which is expected to impact various interactions among small molecules, proteins and DNA/RNA molecules inside bacteria, as well as bacterial interactions with other species, such as bacteriophages."
This work was supported by the University of Arkansas, the Arkansas Biosciences Institute (Grants No. ABI-0189, No. ABI-0226, and No. ABI-0277), and the National Science Foundation (Grant No. 1826642).
About the J. William Fulbright College of Arts and Sciences: Fulbright College is the largest and most academically diverse unit on campus with 19 departments and more than 30 academic programs and research centers. The college provides the core curriculum for all University of Arkansas students and is named for J. William Fulbright, former university president and longtime U.S. senator.
Contacts
Camilla Shumaker, director of science and research communications
University Relations
479-575-7422, camillas@uark.edu