U of A Chemist Develops New Theory for Explaining the Function of Proteins
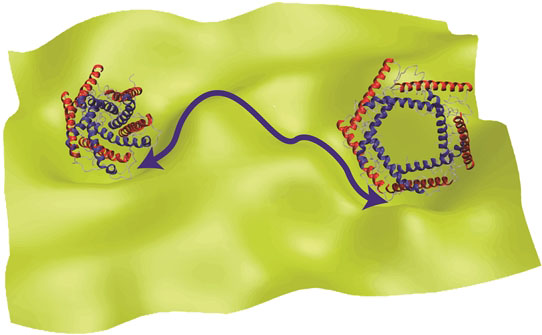
Open and closed forms of channel proteins, which function by changing their shape.
FAYETTEVILLE, Ark. – A University of Arkansas chemist and his collaborator at North Carolina State University have developed a new theory for explaining how proteins and other biomolecules function based on movement and change of shape and structure rather than content.
Proteins are considered the workhorse molecules of cells. They are responsible for nearly all tasks in cellular life, including product manufacture, waste cleanup and routine maintenance. For example, some proteins are responsible for transport of materials and information between the cell and its environment, a vital task for the survival and normal function of the cell. Any disorder in protein function could result in disease, and the study of protein function is necessary for understanding the molecular basis of disease.
“To function, proteins change their shape,” said Mahmoud Moradi, assistant professor of chemistry and biochemistry in the J. William Fulbright College of Arts and Sciences. “Because proteins are not static objects, understanding their conformational dynamics is a necessary step in deciphering the molecular mechanisms underlying their function. The study of protein dynamics is therefore important for understanding the molecular basis of the disease and establishing a ‘rational design’ for developing more efficient drugs.”
The theory developed by Moradi and Ashkan Fakharzadeh, a graduate student North Carolina State University, describes and simulates the way proteins and other biomolecules change their shape to function.
“Conventional theories of protein dynamics ignore the curved nature of the configurational space of biomolecules,” Moradi said. “In this work, we have developed an innovative formalism that relies a geometric theory, traditionally used in general relativity and similar fields, to modify theories of protein dynamics.”
Moradi and Fakharzadeh will address two interrelated questions to further develop their theory: How do proteins function by changing their conformation and by undergoing concerted motions, and how can these conformational changes be simulated at an atomic level? Answering these questions would shed light on the structure-function relationships in proteins, Moradi said, and could improve scientists’ understanding of diseases at a molecular level.
The researchers’ findings were published in the December issue of The Journal of Physical Chemistry Letters, which reports new and original experimental and theoretical research in physical chemistry. A criterion for acceptance in the journal is that the research “reports a significant scientific advance and/or physical insight such that rapid publication is essential.”
About the University of Arkansas: The University of Arkansas provides an internationally competitive education for undergraduate and graduate students in more than 200 academic programs. The university contributes new knowledge, economic development, basic and applied research, and creative activity while also providing service to academic and professional disciplines. The Carnegie Foundation classifies the University of Arkansas among only 2 percent of universities in America that have the highest level of research activity. U.S. News & World Report ranks the University of Arkansas among its top American public research universities. Founded in 1871, the University of Arkansas comprises 10 colleges and schools and maintains a low student-to-faculty ratio that promotes personal attention and close mentoring.
Contacts
Mahmoud Moradi, assistant professor, chemistry and biochemistry
J. William Fulbright College of Arts and Sciences
479-575-6459,
moradi@uark.edu
Matt McGowan, science and research communications officer
University Relations
479-575-4246,
dmcgowa@uark.edu
Headlines
Affairs of the Heart
Find out how biomedical engineering professor Morten Jensen is developing innovative devices to produce better outcomes in cardiovascular medicine.
Students, Faculty and Alumni Kick Off Centennial Year of School of Law
Founded April 14, 1924, the School of Law faculty, students and alumni started the celebration of its centennial year with a Founders Day event and will continue with more commemorative events this coming fall.
Yearly Academic Award Winners, Ambassadors Recognized by Bumpers College
Schyler Angell, Lexi Dilbeck, Cason Frisby, Tanner Austin King, Anna Brooke Mathis, Carrie Ortel, Lucy Scholma, Kadence Trosper and student ambassadors were honored at the college's annual reception.
World Premiere of 'Cries from the Cotton Field' Slated for May 8
Cries from the Cotton Field chronicles the journey of 19th century Italian immigrants from northern Italy to the Arkansas Delta and ultimately to Tontitown. It will premier at 6 p.m. May 8 in Springdale Har-Ber High School.
Fay Jones School's Earth Day Event Spotlights Sustainable Materials and Projects
"One day doesn't seem like a lot, but one day can empower individuals and groups, energize them to work for change and innovate for transformative solutions," professor Jennifer Webb said of the students' design work.




